Drug Establishment Registration is a program that helps ensure the safety and effectiveness of drugs by requiring manufacturers, packagers, and distributors to register their establishments with the FDA. The registration process requires the submission of information about the manufacturing practices, quality systems, and facilities used at an establishment's place of business.
If you're an online retailer and would like to sell pharmaceutical products in the U.S., then you'll most likely have to become FDA Drug Establishment Registered. Once a drug is approved by the FDA, it is assigned a unique identifying code. Once registered, products can be sold in the U.S. without restrictions on advertising or distribution channels.

Drug Establishment Registration Process
Drug Establishment Registration is a process of registering and obtaining a license for the establishment where drugs are manufactured or processed.
This process is conducted by the Food and Drug Administration (FDA) of the Philippines. The purpose of this registration is to ensure that the drugs manufactured in the country are safe for human consumption and are made according to FDA standards.
The applicant must submit an application form along with other requirements such as:
Copy of valid business permit
Proof of tax payment
Proof that they have complied with all requirements of their trade association/association
Drug master file (DME), which contains information about the drug product, including its composition, quality control processes, and manufacturing specifications

Note: Manufacturers must register with the FDA with their own unique Drug Establishment Registration number (DER), which will be used on all labels and packaging for any products they produce. This includes certain types of packaging materials, as well as any ingredients or additives they use in their manufacturing process.
Importers must also register with the FDA, but they do not need to use a DER number on their packaging materials because they do not make the drugs themselves—they just import them from other countries where there may be fewer regulations to ensure quality control.
Every chemical component used as a medicine is considered as a drug under US FDA regulation. This could be used for local application or for oral consumption, or for intravenous usage. It could be for human use or animal use. FDA Drug Facility is any establishment that manufactures, repackages, or re-labels drug products in the United States or foreign location.
Who Need Drug Establishment Registration?
Any company that manufactures, repackages, or re-labels drug products in the United States must register with FDA. The company could be of domestic or foreign origin. Both domestic and foreign establishments must register with FDA. These drug products could be for animal use or human use.
Unless Exempted by law all drug manufactures (Like OTC Drug , Prescription Drugs, Generic Drugs, API, Compounding Drug, Sterilization Facilities, Testing Labs ) must complete FDA Establishment registration within 5 days of beginning of operations.
In Addition to Establishment Registration facilities are required to list their drugs with FDA and this listing needs to be renewed every year before 31st December.
How to Register for Drug Establishment?
The minimum information needed while registering will be:
- Name of establishment(s) manufacturing or processing the listed drug and the type of operation(s) performed.
- DEA schedule.
- Route(s) of administration.
- Inactive ingredients.
- Marketing information
- Information related to the application or OTC monograph citation number Package size and type.
- Flavor, Color, Active Ingredients.
Electronic Drug Listing (EDL) in the FDA database will also need SPLs
The Structured Product Labelling (SPL) is a document markup standard approved by Health Level Seven (HL7) and adopted by FDA as a mechanism for exchanging product and facility information.
In addition to this, the application needs:
- Unique Ingredient Identifiers (UNII)
- Data Universal Numbering System (D-U-N-S®) Number
- If all this jargon seems too much to handle, let’s make it easy.
Remember: Registering at a drug establishment is an essential step in the distribution of pharmaceutical products. It's an important piece of making sure your business complies with all federal and state laws. In this guide, we're going to discuss why it's so important that you register your establishment as well as what steps are involved in registering.
We are the leading experts in this field for 30 years. Let us handle the process, you can handle the profits.Share your contact information with us and we will revert you asap.
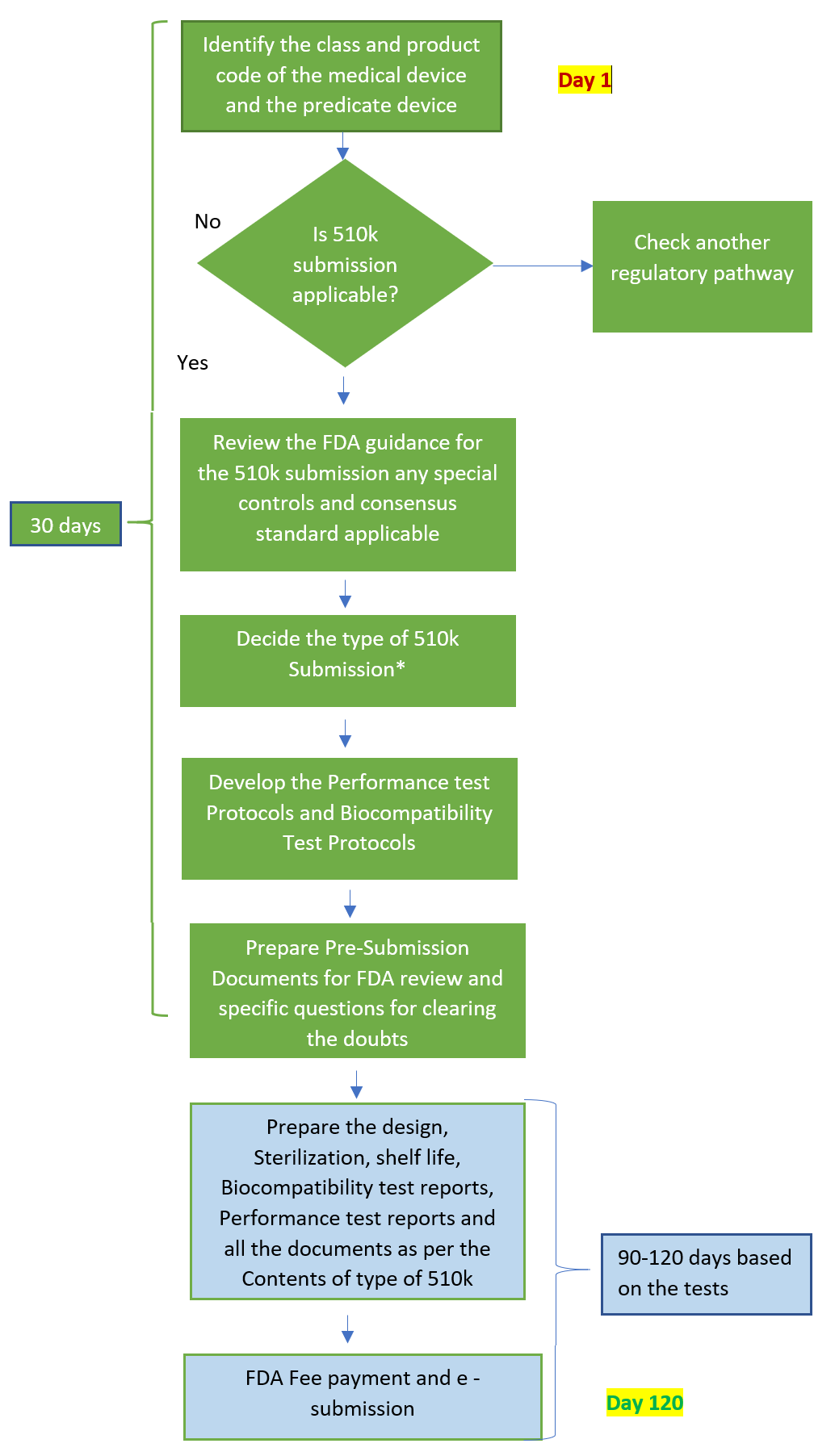
Find more details here at :- 510k submission
No comments:
Post a Comment